Uusi puupylväskirja saatavilla!
13.11.2024
Hot-dip galvanization is the most common surface treatment for steel products. A zinc coating will protect steel from most environmental conditions throughout a product’s lifecycle without requiring any kind of maintenance. Under normal conditions, a hot-dip galvanized product will have a service life of more than fifty years.
As products are dipped in molten zinc during the process, a zinc coating will also form on the inside of tubes and other inaccessible surfaces.

Hot-dip galvanization creates a coating that is highly resistant to dents and scratches, which means that surfaces should not get damaged during transportation or installation. Once installed, these maintenance-free products will not need any retouching.

Hot-dip galvanization is a process in which steel is protected with zinc. All impurities must be removed before the metal is galvanized.
The steel is then cleaned by immersing it in dilute hydrochloric or sulphuric acid. This removes rust and mill cinder.
The item is then rinsed in water before being immersed in a flux bath, where a protective salt layer will form on the surface and prevent the steel from oxidizing before it is placed in the zinc bath.
After cleaning and drying, the item is immersed in molten zinc (450°C). The immersion period typically lasts 3–6 minutes, and will depend on the thickness, size and weight of the item. The zinc and steel react together to form a coating.
The item is then cooled with either air or water before being weighed.
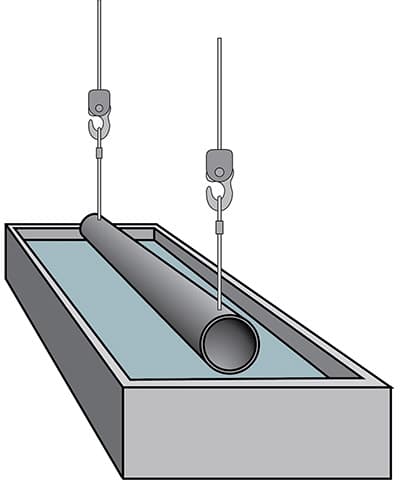
Immersion in acid to remove rust
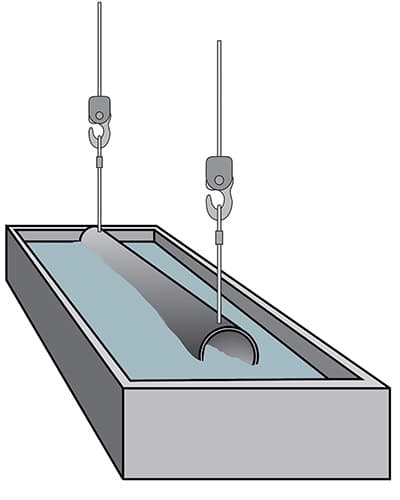
Water rinsing
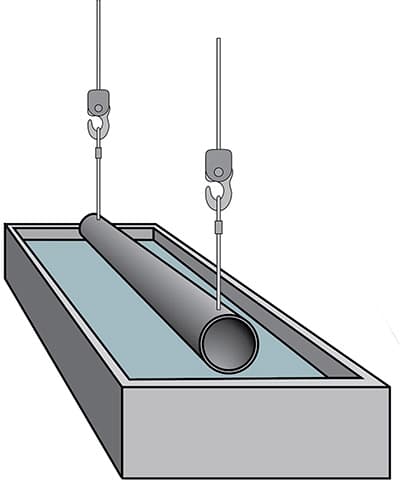
Flux treatment

Immersion in molten zinc
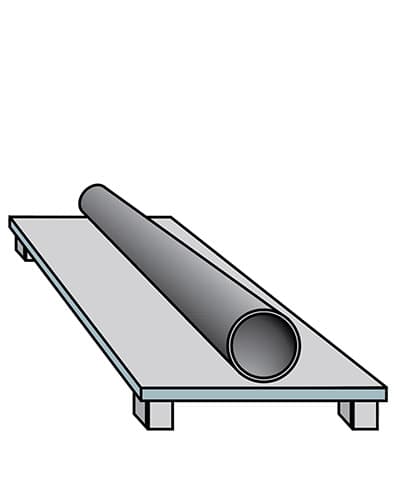
Cooling and finishing
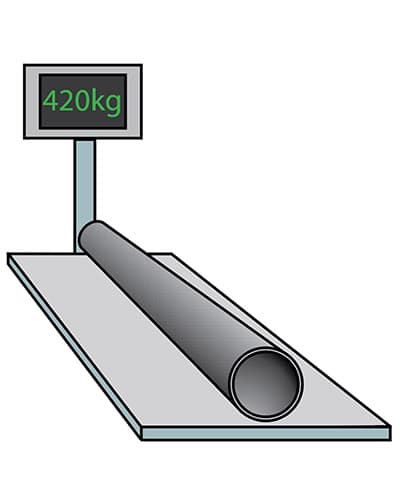
Weighing
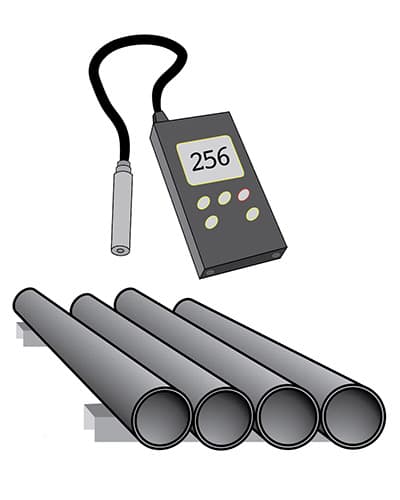
Inspection and measurement


Your choice of steel will have an impact on the quality and appearance of the surface that is formed by the zinc film. Two factors have a fundamental impact on both the appearance of the hot-dip galvanized surface and the thickness and durability of the coating:
The shape and dimensions of the steel structure will affect the immersion method and, thereby, also the end result. The steel’s durability class will not have a direct impact on the appearance or thickness of the zinc coating.
If the appearance of the galvanized steel structure is important, or if the structure will be painted after galvanization, low–silicon steels are recommended (Si + P ≤ 0.04%). This will result in a vibrant, uniformly coloured and long-lasting zinc surface that complies with the SFS-EN ISO 1461 standard. The layers will have an average thickness of 60–90 microns, which is sufficient for climatic conditions in Finland.
Low-silicon steels are subject to limited availability, so this should be taken into account at the design stage.
If a product requires thicker layers of zinc to comply with Finnish categories B or C (more than 100 microns), you must choose a steel with a silicon content of more than 0.15%.
The size of the zinc bath required to galvanize the products and the location of the vent holes should also be taken into consideration at the design stage.
Zinc’s good corrosion resistance is based on the protective layer that forms on its surface (a carbonate layer). The service life of a zinc coating depends on its thickness. According to long-term observations and corrosion tests conducted in Finland, zinc corrodes at an annual rate of about 0.5 micrometres in rural climates and about 1 micrometre in urban climates.
A zinc coating is highly resistant to dents and general wear and tear. But if damage does occur, the iron and zinc will form a galvanic pair in the presence of moisture: the zinc (a base) will act as an anode and the iron as a cathode. The zinc will dissolve around the damaged area and then re-thicken to protect the steel surface. A zinc surface will last dozens – even hundreds – of years.
Immersing steel in molten zinc results in a uniform protective layer. The molten zinc will reach inaccessible inner surfaces and other enclosed spaces. The zinc coating will have a uniform thickness throughout, even at sharp points. Corners that are prone to knocks are protected by more than just a coating of paint.
 The zinc coating is equally thick throughout, even at sharp points.
The zinc coating is equally thick throughout, even at sharp points.
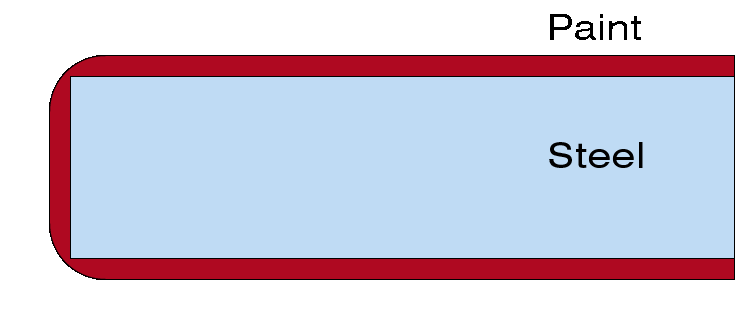
The paint coating is thinner at the sharp points of the part.